COVID drug Molnupiravir expected to hit Chinese market this week

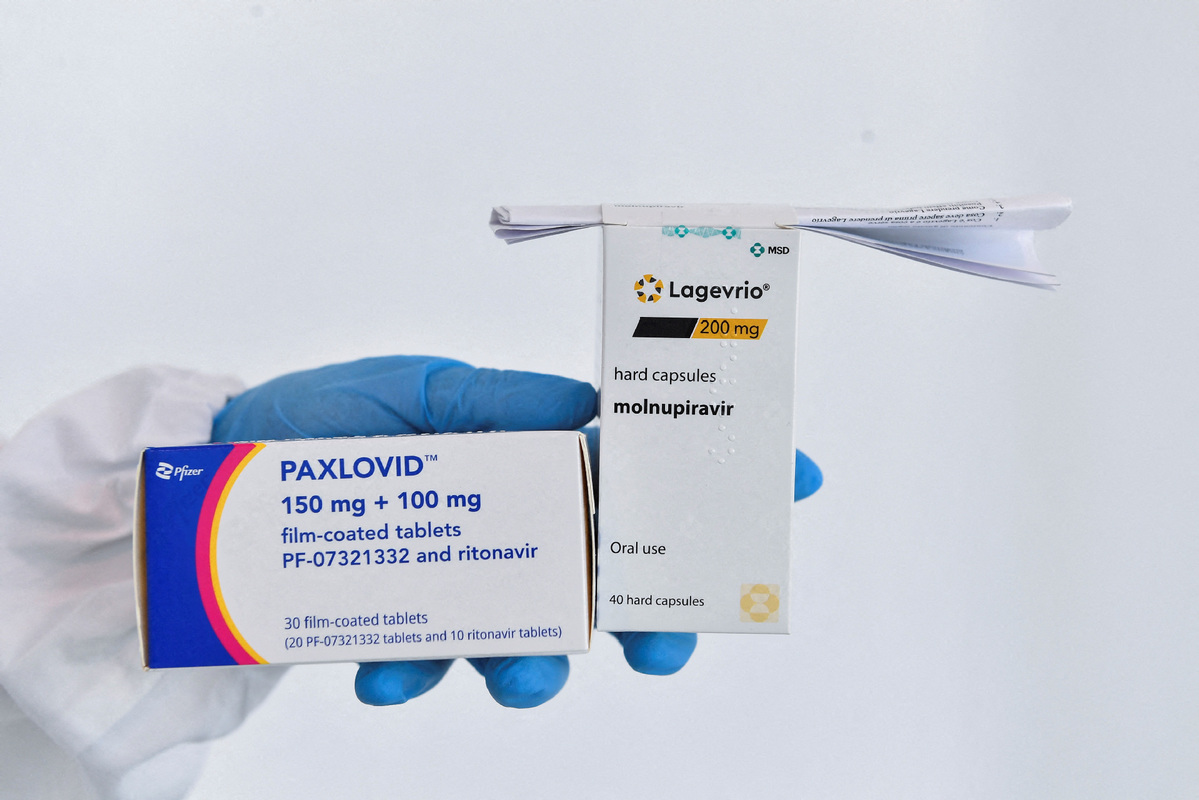
Molnupiravir, a COVID-19 drug developed by US pharmaceutical company Merck is expected to go into market in China on Friday, according to Liu Yong, president of Sinopharm Group.
Liu said Molnupiravir has passed the test for first-time imported drugs on Wednesday and Sinopharm will be responsible for selling the drug in the country, Knews, the news portal of Shanghai Media Group first reported on Wednesday.
The company confirmed with China Daily on Wednesday of the authenticity of the report.
Liu, who is a deputy to Shanghai Municipal People's Congress, made the remarks on Wednesday during the ongoing session of the congress.
The National Medical Products Administration has granted conditional market approval for the import of Molnupiravir on Dec 29, according to a release from the administration published on Dec 30.
The drug can be used for an adult COVID-19 patient who has mild to moderate symptoms and has high risks to develop into severe patients, such as old age, obesity, chronic kidney disease and diabetes, the release said.
- 10 dead and 84 injured in explosion at steel plant
- China unveils flexible urban planning rules to improve lives, foster new industries
- Ex–China Construction Bank executive gets 18 years for bribery, loan violations
- First batch of eco-friendly pioneer zones for construction of beautiful countryside unveiled
- Woodpeckers, finches captured in Jilin winter scenes
- Mainland reiterates 1992 Consensus as foundation for resuming cross-Strait dialogue